Putting a model organism to work
The ER is an organelle that cells in all eukaryotes use to fold proteins, among other things. Under normal conditions, a cell’s need to have proteins folded is balanced by the ER’s capacity to fold them. It’s like driving on a freeway with light traffic, Ko said.
But when cells grow or undergo certain stresses, including attack by pathogens, the demand for protein folding outpaces capacity. This leads to a traffic jam of unfolded proteins. That’s ER stress and, when it gets too severe, it can be fatal.
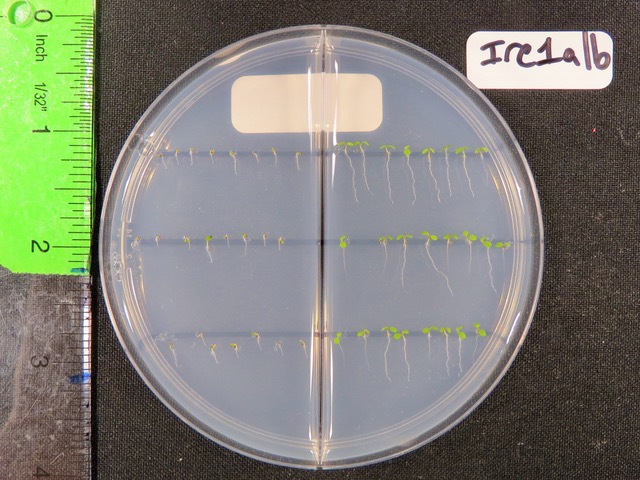
Michigan State University researchers grew a genetic mutant of Arabidopsis thaliana that lacked the IRE1 protein, an important master regulator of endoplasmic reticulum stress. These plants showed growth defects (left) under endoplasmic reticulum stress conditions compared with unstressed conditions (right). The team, however, also showed that further mutations to the IRE1 mutant could restore a plant’s stress response closer to normal. Credit: Dae Kwan Ko/Brandizzi lab
To zero in on one of the pathways that cells use to decide where that tipping point is, the team turned to a model organism, a plant known as Arabidopsis thaliana or thale cress. Using a model like thale cress, scientists can also start identifying genes and traits that are shared, or conserved, in other species.
“These processes are highly conserved, not just in plants, but in animals and all eukaryotes,” Ko said. “Studying these processes in a model system like Arabidopsis has the advantage of letting us carry out research fast using ample genomics resources.”
The team grew “regular” Arabidopsis plants along with other lineages that had random genetic mutations. All told, the team created plants that encompassed thousands of genetic changes.
The researchers then watched how the plants matured after being exposed to a compound that inhibits protein folding. That is, the researchers essentially kickstarted the traffic jam on the ER highway.
While normal plants could withstand this stress, a particular mutant missing a protein known as IRE1 — short for “Inositol Requiring Enzyme 1” — could not. But further mutations to this mutant could return a plant’s ER stress response closer to normal.
“We have this mutant plant that is supposed to be sick under ER stress because it does not have a protein necessary for ER stress responses,” Ko said. “But by mutagenizing the mutant, we found another mutant that reverts the sickness.”
In particular, this more resilient mutant lost an additional protein named PIR1 (short for “Phosphatase type 2CA Interacting Ring finger protein 1”). Working with the Research Technology Support Facility Genomics Core and the Mass Spectrometry and Metabolomics Core facilities at MSU, the researchers also discovered the associated genetics and molecular signaling that determined a cell’s fate in ER stress conditions.

There is more to learn about cell life and death using the Arabidopsis thaliana model organism, Michigan State researchers said. Credit: Alena Kravchenko/Wikimedia Commons
Though this is one pathway in one plant, there’s power in that plant being a model organism like Arabidopsis. The team’s methodology, for example, could be used to look for other important ER stress pathways found in other eukaryotes, such as humans.
And, although PIR1 is only found in plants, it’s found in hundreds of species, including crops like soybeans.
“So, you can start thinking about manipulating the gene activity in plants like soybeans to make them more resilient toward climate change,” Ko said.
“Although PIR1 is not a conserved protein outside the plant kingdom, it is likely that nonplant species use mechanisms similar to those guided by PIR1 to control life-or-death outcomes,” Brandizzi said. “Therefore, our research results can potentially influence research on ER stress management also in nonplant species.”
For Ko, though, there are many other interesting avenues still left to explore in Arabidopsis thaliana itself. For one, plant roots have about a dozen different cell types and understanding if and how this signaling pathway works differently in different cells could have implications for cell health.
“Because the ER is a biosynthetic factory in the cell, understanding how we can manage protein production in the ER has important implications for improving quality of plant biomass and our ability to use plants as large-scale bioreactors for the production of recombinant pharmaceutical proteins, such as antibodies and vaccines,” Brandizzi said.
So, this discovery is a bit like the roots of a germinating plant: Its extent is sure to grow broader and deeper.
This work was supported primarily by the National Institutes of Health (grant number R35GM136637) with contributing support from the Great Lakes Bioenergy Research Center, U.S. Department of Energy Office of Science (DOE-SC), Office of Biological and Environmental Research (grant number DE-SC0018409); DOE-SC, Office of Basic Energy Science, Chemical Sciences, Geosciences and Biosciences Division (grant number DE-FG02-91ER20021); and MSU AgBioResearch (grant number MICL02598).




