About three years ago, Wolfgang “Wolfi” Mittig and Yassid Ayyad went looking for the universe’s missing mass, better known as dark matter, in the heart of an atom.

Their expedition didn’t lead them to dark matter, but they still found something that had never been seen before, something that defied explanation. Well, at least an explanation that everyone could agree on.
“It’s been something like a detective story,” said Mittig, a Hannah Distinguished Professor in Michigan State University’s Department of Physics and Astronomy and a faculty member at the Facility for Rare Isotope Beams, or FRIB.
“We started out looking for dark matter and we didn’t find it,” he said. “Instead, we found other things that have been challenging for theory to explain.”
So the team got back to work, doing more experiments, gathering more evidence to make their discovery make sense. Mittig, Ayyad and their colleagues bolstered their case at the National Superconducting Cyclotron Laboratory, or NSCL, at Michigan State University.
Working at NSCL, the team found a new path to their unexpected destination, which they detailed June 28 in the journal Physical Review Letters. In doing so, they also revealed interesting physics that’s afoot in the ultra-small quantum realm of subatomic particles.
In particular, the team confirmed that when an atom’s core, or nucleus, is overstuffed with neutrons, it can still find a way to a more stable configuration by spitting out a proton instead.
Shot in the dark

Dark matter is one of the most famous things in the universe that we know the least about. For decades, scientists have known that the cosmos contains more mass than we can see based on the trajectories of stars and galaxies.
For gravity to keep the celestial objects tethered to their paths, there had to be unseen mass and a lot of it — six times the amount of regular matter that we can observe, measure and characterize. Although scientists are convinced dark matter is out there, they have yet to find where and devise how to detect it directly.
“Finding dark matter is one of the major goals of physics,” said Ayyad, a nuclear physics researcher at the Galician Institute of High Energy Physics, or IGFAE, of the University of Santiago de Compostela in Spain.
Speaking in round numbers, scientists have launched about 100 experiments to try to illuminate what exactly dark matter is, Mittig said.
“None of them has succeeded after 20, 30, 40 years of research,” he said.

“But there was a theory, a very hypothetical idea, that you could observe dark matter with a very particular type of nucleus,” said Ayyad, who was previously a detector systems physicist at NSCL.
This theory centered on what it calls a dark decay. It posited that certain unstable nuclei, nuclei that naturally fall apart, could jettison dark matter as they crumbled.
So Ayyad, Mittig and their team designed an experiment that could look for a dark decay, knowing the odds were against them. But the gamble wasn’t as big as it sounds because probing exotic decays also lets researchers better understand the rules and structures of the nuclear and quantum worlds.
The researchers had a good chance of discovering something new. The question was what that would be.
Help from a halo
When people imagine a nucleus, many may think of a lumpy ball made up of protons and neutrons, Ayyad said. But nuclei can take on strange shapes, including what are known as halo nuclei.
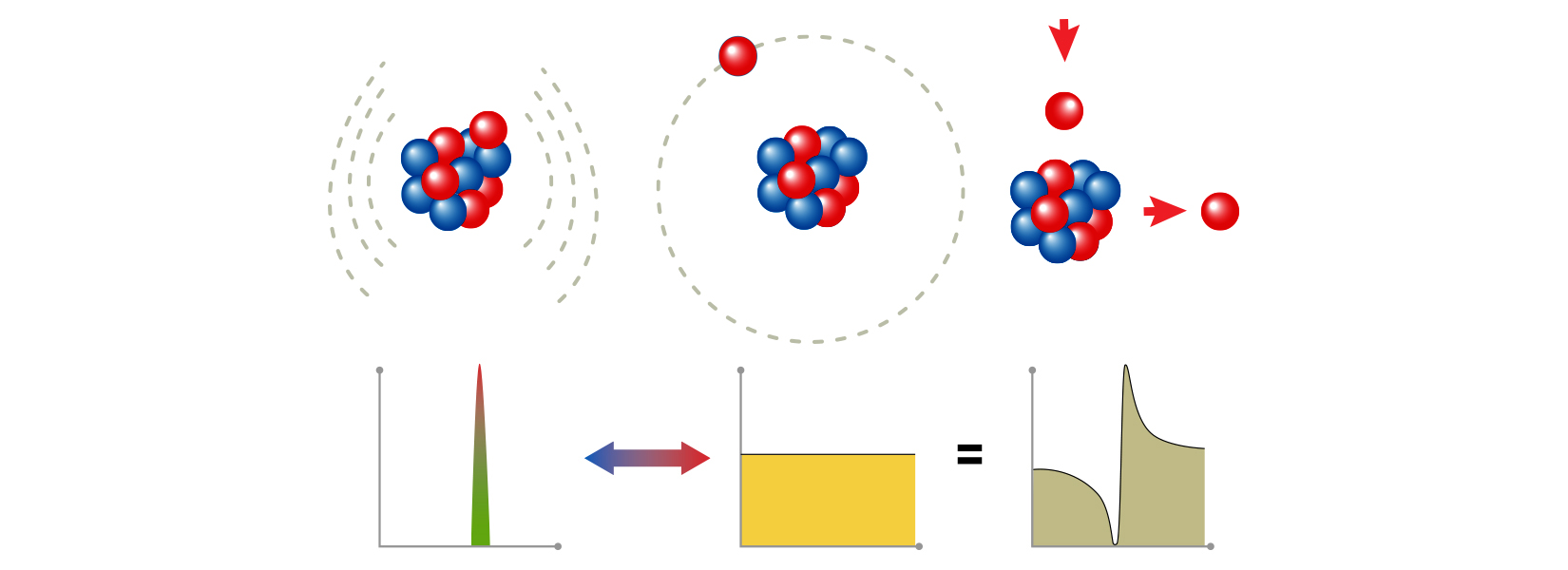
Beryllium-11 is an example of a halo nuclei. It’s a form, or isotope, of the element beryllium that has four protons and seven neutrons in its nucleus. It keeps 10 of those 11 nuclear particles in a tight central cluster. But one neutron floats far away from that core, loosely bound to the rest of the nucleus, kind of like the moon ringing around the Earth, Ayyad said.
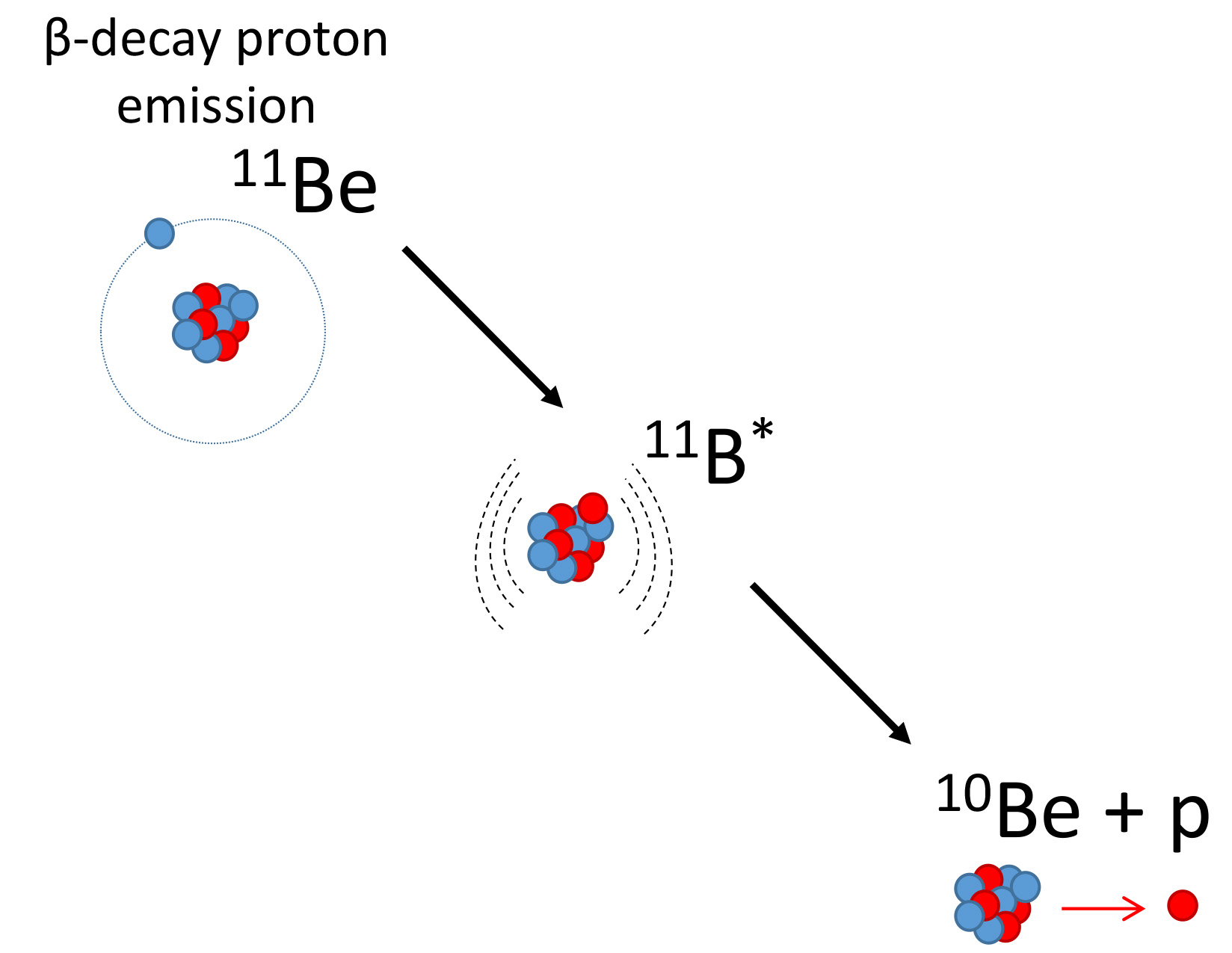
Beryllium-11 is also unstable. After a lifetime of about 13.8 seconds, it falls apart by what’s known as beta decay. One of its neutrons ejects an electron and becomes a proton. This transforms the nucleus into a stable form of the element boron with five protons and six neutrons, boron-11.
But according to that very hypothetical theory, if the neutron that decays is the one in the halo, beryllium-11 could go an entirely different route: It could undergo a dark decay.
In 2019, the researchers launched an experiment at Canada’s national particle accelerator facility, TRIUMF, looking for that very hypothetical decay. And they did find a decay with unexpectedly high probability, but it wasn’t a dark decay.
It looked like the beryllium-11’s loosely bound neutron was ejecting an electron like normal beta decay, yet the beryllium wasn’t following the known decay path to boron.
The team hypothesized that the high probability of the decay could be explained if a state in boron-11 existed as a doorway to another decay, to beryllium-10 and a proton. For anyone keeping score, that meant the nucleus had once again become beryllium. Only now it had six neutrons instead of seven.
“This happens just because of the halo nucleus,” Ayyad said. “It’s a very exotic type of radioactivity. It was actually the first direct evidence of proton radioactivity from a neutron-rich nucleus.”